
In a breakthrough discovery, scientists from VIB-VUB Center for Structural Biology unraveled the mysteries of SlyB, a tiny but mighty protein found in the outer membrane of certain bacteria. When bacteria face stress, SlyB acts as a crucial guardian to protect the bacterial cell from dying. This discovery not only deepens our understanding of bacterial survival mechanisms but also paves the way for potential applications in antimicrobial research. The findings are published in Nature.
Bacteria on the WHO blacklist
Biologists categorize bacteria into two major groups: Gram-positive bacteria, characterized by a single membrane, and Gram-negative bacteria, distinguished by an inner and outer membrane. It is this second membrane that makes the Gram-negative bacteria extra hard to treat. The outer membrane forms an essential protective layer that shields cells from environmental stresses such as antimicrobial peptides and antibiotics. The World Health Organization (WHO) has a top 10 list of the most worrying pathogens. Unsurprisingly, this list is predominantly populated by Gram-negative bacteria. Scientists have eagerly sought vulnerabilities in these double-layered bacteria and now, a team of Belgian researchers at VIB-VUB Center for Structural Biology has made a breakthrough.
Stress conditions in the membrane
Although forming a protective shield to the bacteria, the outer membrane is not failsafe. When under assault from external stressors, the phospholipids in the outer membrane clutter together like oil spills on the cell surface. This creates weak zones in the shield that risk to burst under the water pressure in the cells, resulting in a blow-out of the cell content and bacterial death through a process called lysis.
SlyB: the bacterial lifebuoy
Enter SlyB, a protein with a function previously unexplored in science. When cells sense membrane weakening stresses, SlyB comes into play. The protein moves to the phospholipid spills, where it creates ring-shaped nanodisc structures - akin to a lifebuoy. The SlyB nanodiscs encapsulate the proteins in the compromised zones of the outer membrane. This SlyB activity protects the proteins and reinforces the weaker sections of the outer membrane, preventing the excessive loss of essential proteins and ensuring the overall health and survival of the bacteria. Without SlyB the membrane becomes punctured and cells bleed out and lyse. “With this discovery, we have gained a profound new insight into how Gram-negative bacteria protect themselves from membrane destabilizing conditions” says Arne Janssens, PhD student at VIB-VUB Center for Structural Biology.
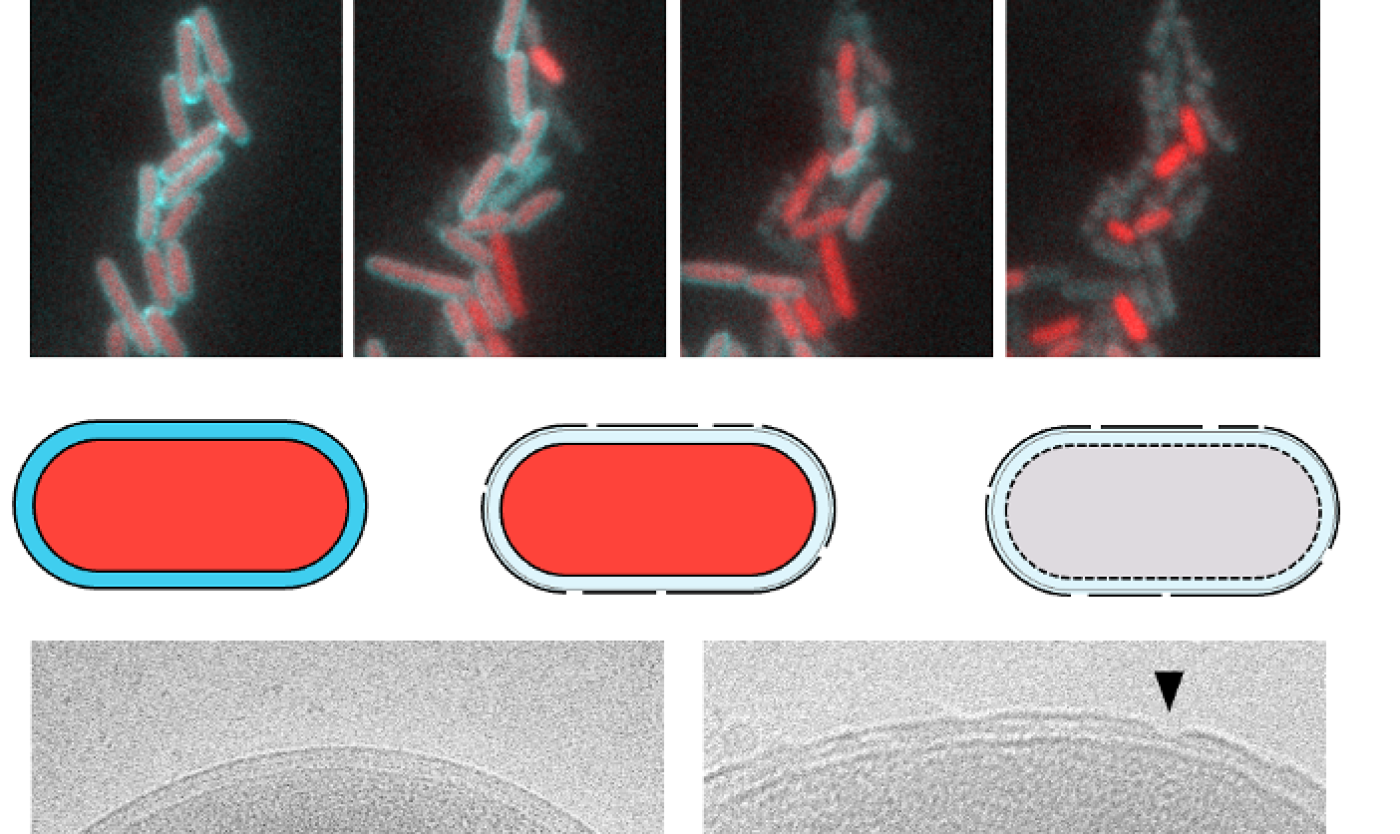
Microscopic snapshots and schematic drawing of bacterial cells exposed by antimicrobial peptides. Without the SlyB protein, the outer membrane weakens and cell contents leak out of the cells, resulting in cell lysis. Electron microscopy images below left and right show the healthy and damaged membranes in Gram-negative bacteria.
Future applications
The study not only brings fundamental insights in a novel family of nanodisc-forming proteins, it also provides new tools to fight the bacteria. The Belgian researchers are already progressing to the next steps by using SlyB’s unique properties to their own benefit. The nanodiscs with bacterial membrane proteins are purified and used as vaccination vehicles to stimulate the immune system so it can recognize incoming threats better. The discovery also creates perspectives in the generation of new drugs that prevent Gram-negative bacteria from forming the nanodiscs, rendering them unable to fence off assaults. “Focusing on these nanodiscs, we aim to turn the bacteria’s protection strategy into its Achilles heel,” says Prof. Han Remaut, Group Leader at VIB-VUB Center for Structural Biology. “By doing so, we discover novel methods to combat Gram-negative bacteria and ultimately shorten the WHO's list of concerning pathogens.”
The study is a collaboration between scientists from Flanders Institute for Biotechnology (VIB), VUB, UGent, UCL Louvain-La-Neuve, and Colorado School of Mines (USA).